The quest for advanced battery technologies has become a focal point in both academic and industrial research due to the heightened demand for energy storage solutions in electric vehicles, portable electronics, and renewable energy systems. Notably, researchers are concentrating on improving batteries’ energy density, charging speed, discharging rates, and overall lifespan. One area that shows considerable promise is the development of layered lithium-rich transition metal oxides, which have emerged as potential game-changers in battery performance.
Layered lithium-rich transition metal oxides are gaining attention largely because their unique structural properties offer advantages that could lead to superior battery performance. The fundamental capacity of these cathodes to facilitate lithium migration during charging and discharging processes allows for enhanced energy storage. Their lithium-rich compositions enable the storage and release of greater energy quantities, making them particularly appealing for applications that require high energy resilience, like electric vehicles and high-performance smartphones.
Furthermore, these cathodes integrate transition metals, such as manganese, cobalt, or nickel, along with oxygen anions. Together, these components create a favorable environment for redox reactions—essential processes that drive the electron flow necessary for energy generation in batteries.
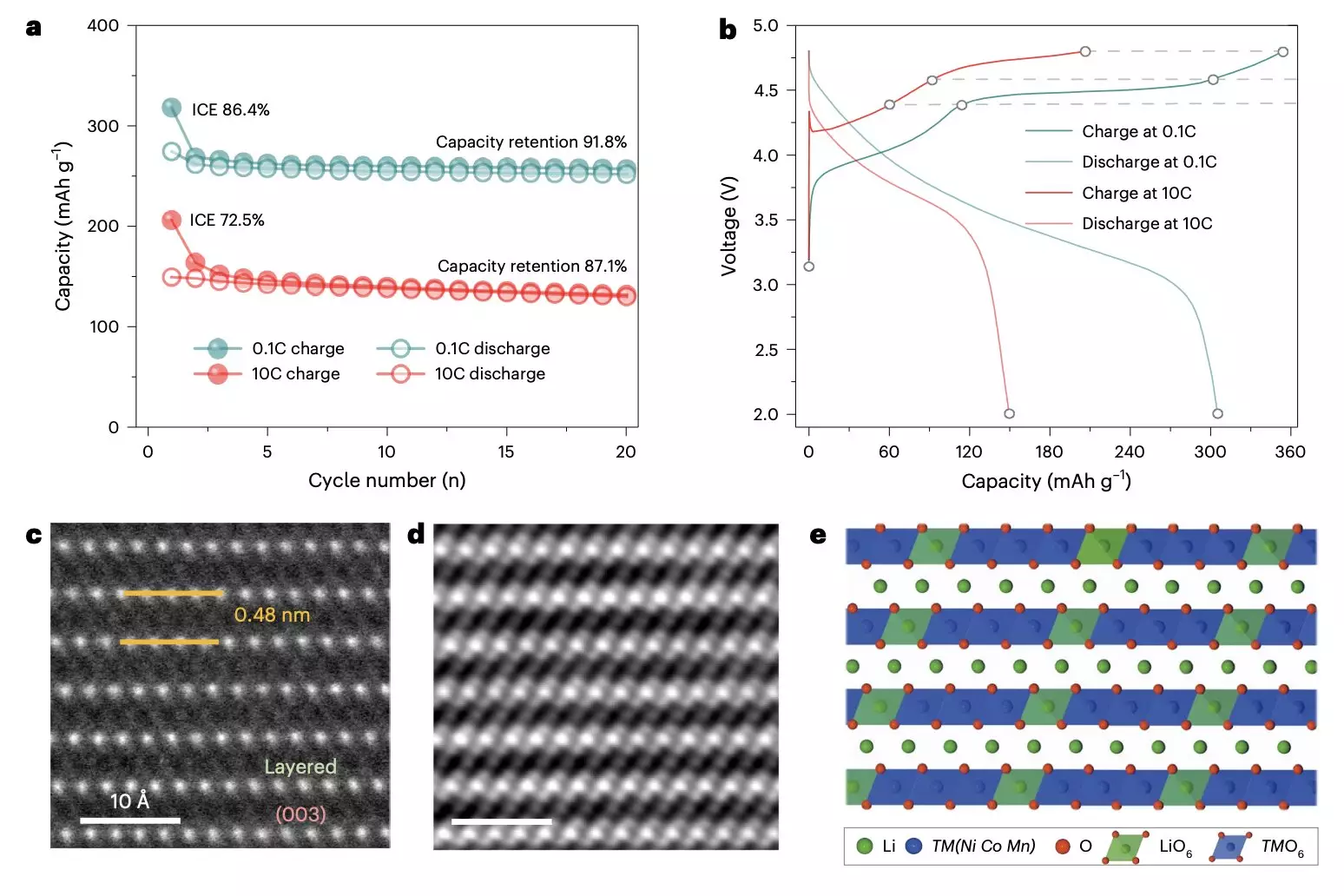
Despite the advantages, the journey towards widespread adoption of layered lithium-rich metal oxides is fraught with challenges, primarily their propensity for rapid degradation over time. This leads to diminished voltage capacity and operational lifespan, posing significant barriers to their extensive use. Researchers from Sichuan University and Southern University of Science and Technology have recently investigated the intricacies behind this degradation phenomenon. Their findings, published in *Nature Nanotechnology*, examine how various structural and chemical factors contribute to this critical issue.
The team’s comprehensive analysis integrates information at both atomic and particle levels, employing intricate techniques like energy-resolved transmission X-ray microscopy (TXM) to scrutinize the cathodes closely. Through this advanced imaging, they discerned essential oxygen defects and distortions that emerge at different charging rates, signaling pathways leading to degradation.
The researchers articulated that the degradation process often begins with the formation of numerous oxygen defects due to slow electrochemical activation. These defects instigate a series of detrimental transformations within the cathode structure, leading to the formation of nanovoids and significant changes in lattice structure. Key observations included ultrafast lithium movements that caused distortions in the lattice, as well as the dissolution of transition metal ions, which further complicates the material’s integrity.
As a result, these irreversible transformations, characterized by particle cracking and suboptimal cycle efficiency, underline the importance of understanding the internal mechanics at play. They contribute to inconsistent performance and ultimately curtail the cathode’s effective lifespan.
The insights gleaned from this groundbreaking study are of paramount importance for future research and development in battery technologies. Understanding the complex interactions and reactions that lead to degradation can pave the way for innovative strategies aimed at mitigating these undesirable effects.
For instance, future research may focus on alternative materials or structural modifications that could minimize the formation of oxygen defects. Developing enhanced synthesis techniques or stabilizing agents to reinforce the integrity of layered lithium-rich cathode structures is also a potential avenue for exploration.
While layered lithium-rich transition metal oxides demonstrate exceptional potential in revolutionizing battery performance, the challenge of degradation cannot be overlooked. By delving deep into the structural and chemical factors that contribute to this complexity, researchers are in a position to forge new paths for the creation of next-generation batteries. As this field continues to evolve, the findings of studies such as that conducted by the research teams from Sichuan University and Southern University of Science and Technology will remain crucial in steering the course for more efficient and durable energy storage solutions.