In the quest for sustainable energy sources, the transformation of carbon dioxide (CO2) into usable fuels presents a promising avenue. A groundbreaking artificial photosynthesis system developed by researchers at the University of Michigan demonstrates a compact yet powerful capability to synthesize hydrocarbons, particularly ethylene, through an innovative method that stands out among existing technology. Ethylene production is notable not only for its central role in the creation of plastics, one of the most widely produced organic compounds globally, but also for the potential environmental benefits associated with its production from CO2 that would otherwise contribute to atmospheric pollution.
Understanding the Process of Artificial Photosynthesis
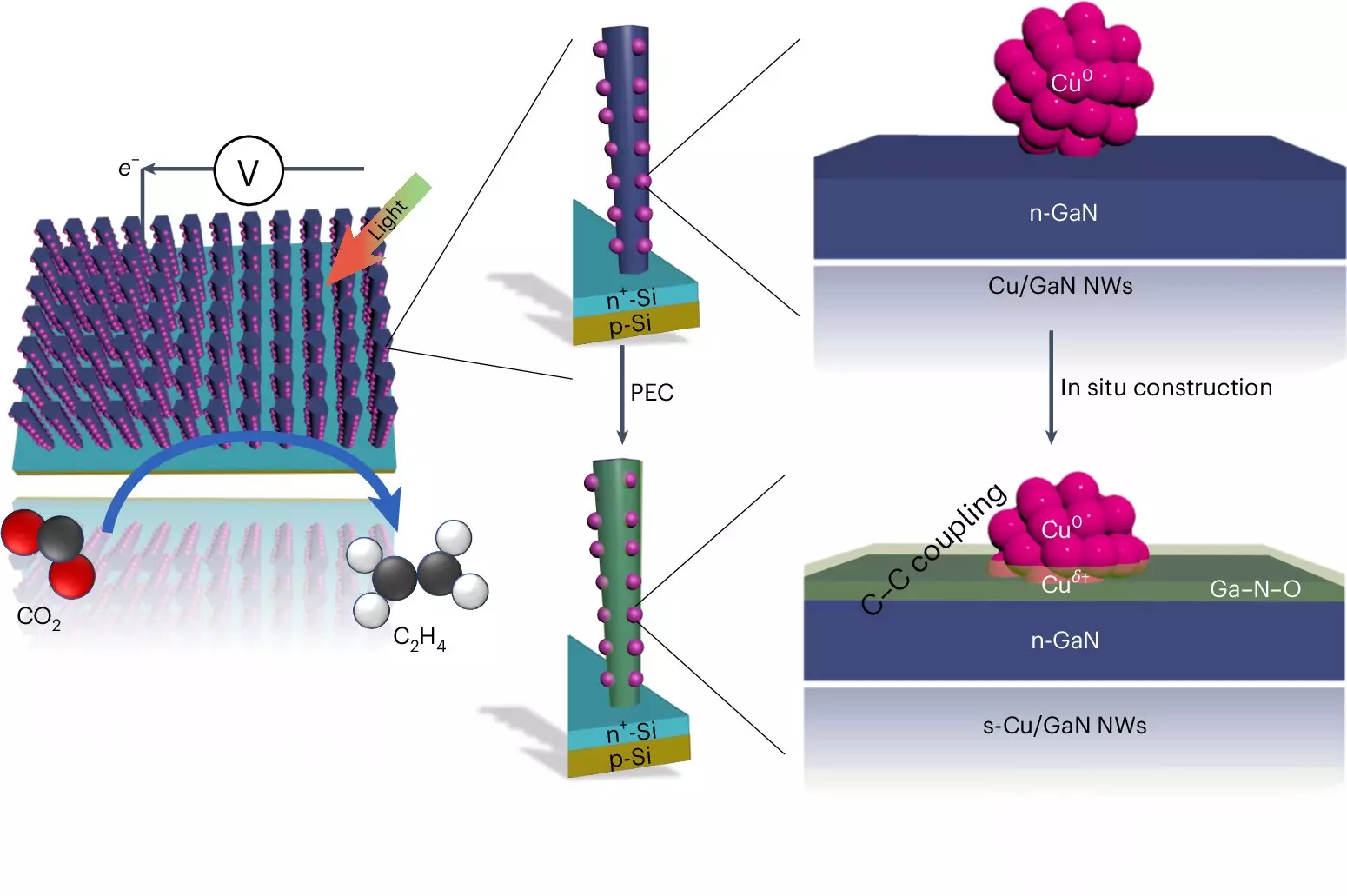
This novel approach leverages the principles of photosynthesis, which enables plants to convert sunlight, CO2, and water into energy-rich compounds. The researchers optimized their system to achieve highly efficient and stable performance, boasting activity and longevity figures significantly superior to conventional systems that reduce CO2 using solar energy. According to Professor Zetian Mi, the key to their success lies in the combination of an intricate setup involving gallium nitride nanowires and advanced catalyst materials. This combination allows for enhanced interaction with light and maximizes the reaction efficiency.
The process begins with the absorption of sunlight, which energizes electrons in the gallium nitride nanowires—ultra-fine structures measuring only 50 nanometers in width. This electron excitation enables the splitting of water molecules into hydrogen and oxygen. It is this liberated hydrogen that interacts with CO2 to facilitate the transition towards hydrocarbons.
What sets this artificial photosynthesis system apart is its remarkable efficiency. Studies have shown that approximately 61% of the free electrons generated are effectively utilized in the conversion reaction to produce ethylene, positioning this technology as a leader in the field. In contrast to other catalysts, which often falter after brief operation periods, the University of Michigan’s device demonstrated endurance over extended durations—running for 116 hours without a decline in performance and surpassing 3,000 hours in similar configurations.
The foundational element in this stability is the twice-functional resilience of the gallium nitride. The interaction of oxygen production, a byproduct of water splitting, leads to a self-healing mechanism within the catalyst. This natural maintenance significantly contributes to the improved longevity—a critical consideration for any commercial application of such technology.
The team aims not merely to maximize ethylene output; their broader vision encompasses the synthesis of multi-carbon compounds such as propanol—a three-carbon alcohol that also holds potential in the fuel market. The implications of this research are vast, extending into various sectors dependent on liquid fuels which could markedly reduce reliance on fossil fuels and mitigate greenhouse gas emissions.
The pathway to accomplishing this ambitious goal involves continued exploration of the device’s limitations and potential enhancements. Researchers must discover ways to manage the reaction environment effectively while promoting further carbon chain elongation to develop higher-order hydrocarbons. Success in this area could catalyze a shift towards more sustainable methods for producing fuel derivatives, making existing transportation technology more eco-friendly.
The work conducted at the University of Michigan exemplifies the intersection of cutting-edge research and practical applications that could one day redefine how we produce and consume energy. By harnessing carbon emissions and using them as building blocks for essential fuels and materials, society could significantly reduce its carbon footprint and shift towards more sustainable energy paradigms.
As we delve deeper into this innovative research, it becomes increasingly clear that scientists and engineers play an essential role in addressing global environmental challenges. The integration of renewable energy perspectives and advanced materials science into creating functional systems signals a hopeful pathway ahead—a future where our waste products are not simply discarded but instead transformed into valuable resources that benefit our society and planet at large. Through continuous improvement and cooperation in research, we can pave the way towards a cleaner, more sustainable future in global energy production.