Transcranial focused ultrasound (TFUS) is emerging as a groundbreaking non-invasive technique in the realm of neurology, offering a new frontier in the treatment of neurological disorders. By employing high-frequency sound waves, researchers are harnessing this technology to stimulate targeted regions of the brain, opening the door for innovative treatments for conditions such as drug-resistant epilepsy and tremors. The latest advancements in this field have been bolstered by a collaborative effort among researchers from Sungkyunkwan University (SKKU), the Institute for Basic Science (IBS), and the Korea Institute of Science and Technology.
The crux of the recent research revolves around the development of a novel sensor detailed in a publication in *Nature Electronics*. This cutting-edge device not only records neural signals but also stimulates specific areas of the brain with low-intensity ultrasound waves. The significance of this innovation cannot be overstated, particularly for patients suffering from severe neurological conditions where traditional treatment modalities have proven ineffective.
Challenges in Conventional Brain Sensors
One of the critical challenges in existing brain sensor technologies has been their inability to effectively measure signals from the complex and highly contoured surfaces of the brain. Previous sensor designs, including those developed by notable researchers like Professors John A. Rogers and Dae-Hyeong Kim, faced significant limitations. While these sensors exhibited a thinner profile that contributed to improved signal collection, they often struggled to maintain adhesion on regions with steep curvatures—a common feature in human brains.
Donghee Son, a leading figure in the current study, underscored this issue by stating that the lack of a secure fit on the brain’s surface hindered accurate diagnostics and reliable measurements. Consequently, the gap remained in the ability to achieve prolonged, continuous data collection crucial for personalized treatment strategies.
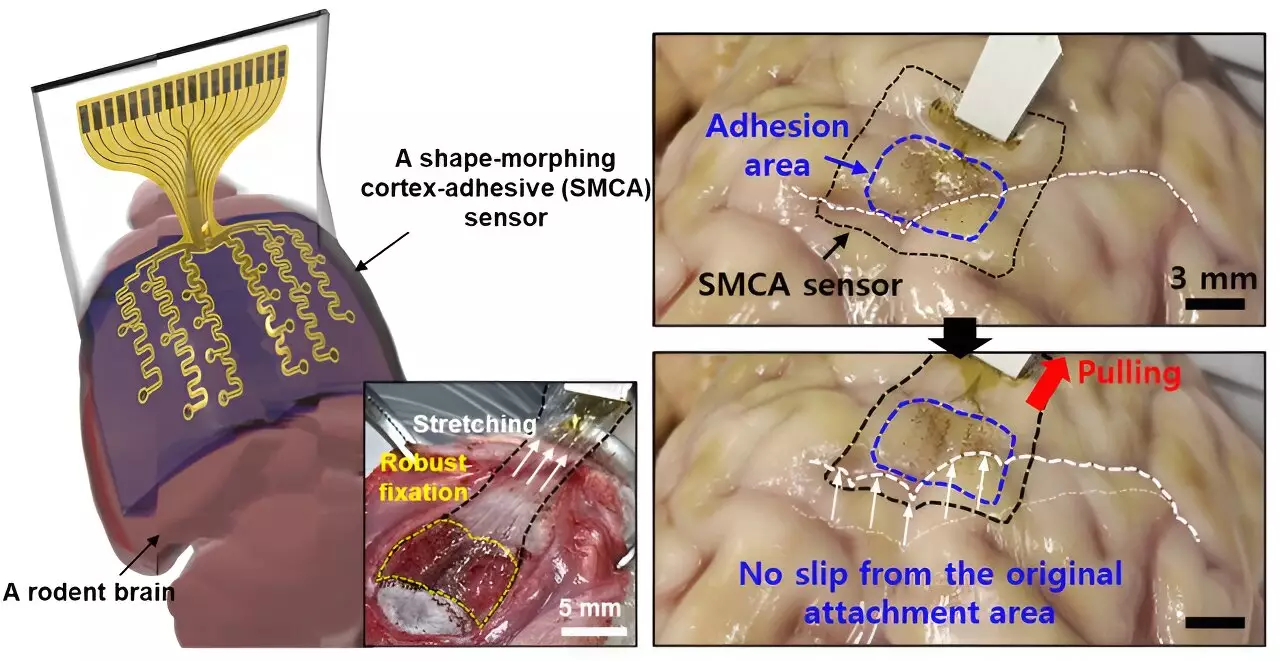
In response to these limitations, the research team has introduced an innovative sensor known as ECoG (electrocorticography). Unlike its predecessors, the ECoG sensor is designed to adapt its shape, allowing it to tightly follow the contours of the cortical surface. “The new sensor we developed can tightly conform to highly curved brain regions and adhere firmly to the brain tissue,” Son remarked, highlighting the potential for enhanced measurement reliability.
A standout feature of the ECoG sensor is its robust adhesion, which minimizes external mechanical noise that can disrupt precise neurological measurements. As noted, maintaining the integrity of these signals is essential, especially in the context of managing epilepsy, where external vibrations and fluctuations in brain activity can severely impede treatment efficacy.
Real-Time Monitoring and Personalized Treatments
The success of tailored therapeutic interventions relies heavily on real-time monitoring of brain activity. In conventional sensor setups, the noise generated by ultrasound vibrations often obscured critical brain wave data, which hampered efforts to create individualized treatment plans. However, with the ECoG sensor’s ability to reduce such noise significantly, the potential exists to refine treatment protocols based on real-time feedback.
Personalized ultrasound stimulation treatments have gained traction in recent years, with an increasing number of research teams striving to cater to individual patient needs. The ECoG technology addresses key challenges associated with conventional sensors, thus paving the way for more targeted and effective treatment strategies for disorders like epilepsy.
The ECoG sensor comprises a sophisticated three-layer structure that demonstrates advanced medical engineering. The first layer, constructed of a hydrogel-based material, forms a bond with the brain tissue, ensuring a secure fit. The second layer features a self-healing polymer that allows for shape morphing, enabling the sensor to tailor itself to the intricate complexities of the brain’s surface. Finally, a stretchable and ultra-thin layer containing gold electrodes facilitates direct signal measurement.
Once the hydrogel layer begins to gel upon application, strong adhesion to the brain tissue is initiated, allowing for long-term use without dislodgment. This breakthrough opens new avenues for extended monitoring and treatment protocols, beneficial for patients requiring chronic interventions.
Future Directions and Potential Applications
The efficacy of the ECoG sensor has been demonstrated in initial tests conducted on awake, living rodent models, yielding encouraging results in both measuring brain waves and managing seizure activities. Beyond immediate applications for epilepsy treatment, the versatility of the ECoG sensor suggests that it could be instrumental in diagnosing and addressing a variety of neurological disorders.
Future plans for the sensor include scaling its design to incorporate a high-density array of electrodes, enhancing the resolution of data collection. As this technology undergoes clinical trials, its successful implementation could revolutionize approaches to brain health—benefiting not only patients suffering from epilepsy but also those with a spectrum of neurological conditions.
The novel ECoG sensor exemplifies the next generation of brain-computer interfaces, where innovation merges with clinical need. The continual improvement of such technologies has the potential to redefine how neurological disorders are diagnosed and treated, elevating the standards of patient care in the field of neurology.