Advancements in scientific research often emerge from the intersections of different fields, prompting innovative perspectives on longstanding biological mysteries. A significant example of this convergence is found in the recent study conducted by researchers at São Paulo State University (UNESP) in Brazil. Their exploration into protein compartmentalization within cells demonstrates the utility of classical mixture theory from condensed matter physics, ultimately proposing a Griffiths-like phase applicable to cellular behaviors.
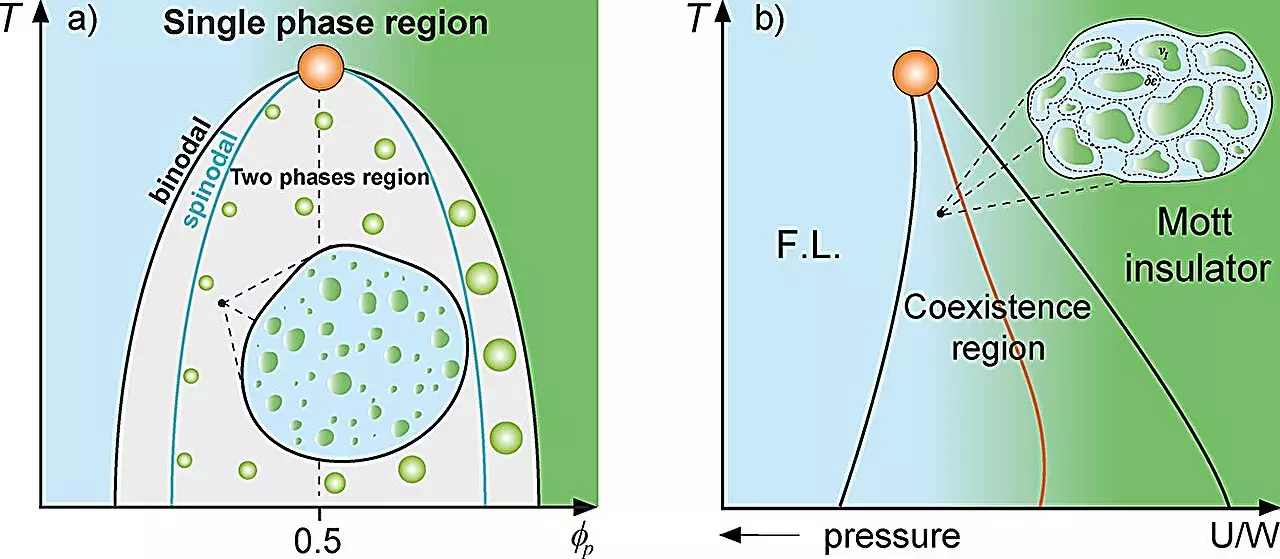
Protein compartmentalization, a fundamental aspect of cellular structure and function, can sometimes resemble phenomena observed in physical systems. In traditional mixture theory, the interactions between different phases can lead to various emergent properties. The UNESP team’s examination of protein droplets within cellular environments reflects a similar complexity, drawing analogies to the Griffiths phase found in magnetic systems. Simply put, just as magnetic properties manifest in regions of differing magnetization, cellular dynamics can also exhibit varied behaviors depending on the molecular composition and interactions at play.
The study, spearheaded by Ph.D. candidate Lucas Squillante and guided by professor Mariano de Souza, investigates how high concentrations of proteins lead to liquid-liquid phase separation. This vast segregation results in the formation of droplets that serve as unique cellular environments. Using thermodynamic models—such as the Flory-Huggins model known for describing polymer solutions—the researchers illustrate how these protein-rich droplets affect cellular dynamics, specifically near the crucial binodal line that dictates phase separation.
The Concept of Rare Regions in Cellular Context
A critical aspect of the GNU framework is the emergence of “rare regions,” akin to those observed in magnetic Griffiths phases. In the cellular context, these rare regions manifest as protein droplets that alter local dynamics. Through thermodynamic analysis, the researchers assert that the fluctuations in protein movement can be dramatically reduced in these droplets, echoing the behavior seen in magnetic systems where non-magnetized or magnetized regions interact with their environments. As the dynamics shift in these cellular “rare regions,” it potentially sets the stage for significant changes in overall cellular behavior, including gene expression optimization.
Moreover, rather fascinatingly, this research advances beyond mere cellular function to contemplate life’s origins. The study suggests that this Griffiths-like phase may have played a pivotal role in the emergence of primordial organisms. In a historical context, this aligns with the classical theories put forth by Russian biologist Aleksandr Oparin on coacervates—droplets necessary for early biological processes to evolve.
Another critical insight from the research is the relationship between protein dynamics and homochirality, the predominance of a single chirality in biological molecules, which is vital for the proper functioning of proteins. The authors discuss how the slow dynamics associated with these protein droplets may influence molecular interactions crucial for biological evolution. Chirality derives from a molecule’s non-superimposable nature on its mirror image, much like human hands. As such, understanding how homochirality participates in genetic expression can unveil significant evolutionary advantages, potentially helping in the genesis of more complex biological systems.
In exploring the dynamics of proteins within their cellular microenvironments, the researchers emphasize the dynamic relationship between protein behavior and the broader implications for life as we know it. This connection to chirality may offer avenues for new research into how life’s building blocks are organized and optimized.
Implications for Disease Understanding and Treatment
The implications of this line of inquiry extend deeply into medical research as well. The understanding of liquid-liquid phase separation is gaining traction in the study of various diseases, including neurodegenerative disorders and cancer. The researchers highlight that compartmentalization through phase separation can influence how proteins related to diseases function within cellular environments, potentially impacting mutation processes and disease progression.
For instance, aberrations in protein compartmentalization can be observed in conditions such as cataracts or the responses to COVID-19, where the behavior of specific proteins can suppress immune responses. Importantly, as proposed by co-author Marcos Minicucci, insights into this Griffiths-like cellular phase could inform therapeutic strategies, ushering in novel treatment pathways for a range of conditions driven by protein dynamics.
By marrying concepts from condensed matter physics with those of molecular biology, the UNESP researchers encapsulate the interdisciplinary nature of modern scientific research. Their work not only enriches our understanding of cellular phenomena but also opens potential pathways for future research into the evolution of life and disease management, reinforcing the notion that knowledge across disciplines can drive profound developments in both fundamental science and applied medicine.